Chemical physics is a scientific discipline which utilizes physical methods to gain insight into the nature of molecules, their interactions, dynamics, etc. This encompasses the study of molecules from the smallest ones as for instance water to macromolecules (polymers) and their complexes and systems (solutions, suspensions, gels, polymer melts). The chemical physics research area is very close to the concept of soft matter physics. This department devotes itself mainly to polymers, supramolecular structures, and self-assembled mesoscale structures composed of either polymers or low molecular mass compounds. While biological polymers (nucleic acids, proteins, polysaccharides) are an integral part of living organisms and have been on Earth for a long time, synthetic polymers saw the light of day at the beginning of the 20th century. Although the first synthetic polymer (bakelite) was publicly introduced in 1907, the concept of the polymer, defined as long chains of atoms held together by covalent bonds, emerged considerably later (1920), Hermann Staudinger, later Nobel Prize laureate). The real development of polymer chemistry and physics occurred much later in the 20th century (Nobel Prizes – chemists Giulio Natta and Karl Ziegler, 1963, physicists Paul Flory, 1974 and Pierre-Gilles de Gennes, 1991 and physical chemists Alan J. Heeger, Alan G. MacDiarmid and Hideki Shirakawa, 2000). Polymeric macromolecules demonstrate such properties and behavior that bear no resemblance to small “classical” molecules. Although research studies of synthetic and biological polymers were carried out either in parallel or independently, it turned out that these polymers exhibit many common phenomena and properties. Our department focuses especially on ionic polymers (polyelectrolytes). The presence of charges in these molecules enables water solubility, ecological applications, and biological functions of biopolymers. On the other hand the presence of charges results in strong electrostatic interactions and complicates understanding of their structure, dynamics, properties, polymerization kinetics, etc.
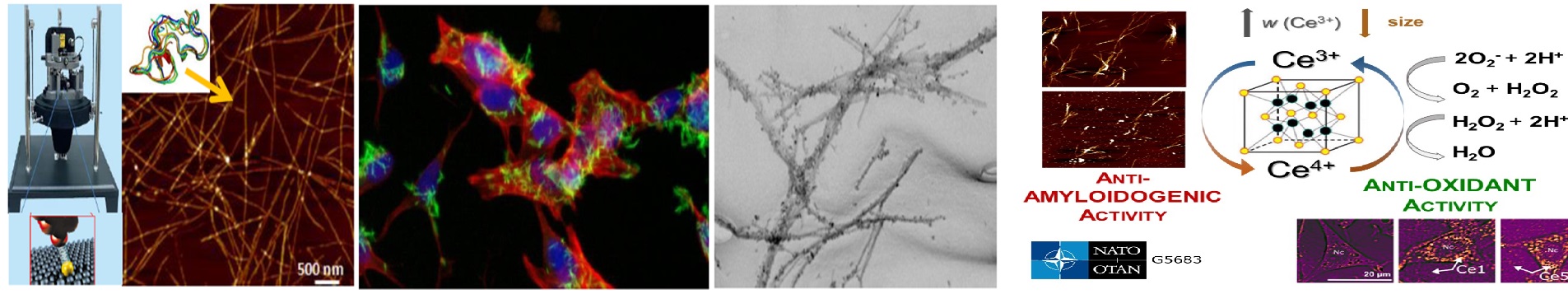
Macromolecules and their systems (solutions), especially in the case of ionic macromolecules represent a complex multiparticle problem for physicists. Multiparticle collective interactions cannot be measured directly, and therefore the information on the structure and dynamics of these systems gained experimentally in our department contribute to our knowledge about them. In the recent past, we have succeeded in reorienting our long-term basic research findings also towards applications. We have created and patented a new mechanism of polymer nanoparticles formation with adjustable parameters based on physical bonds (not chemical reactions). Polymer nanoparticles find applications in many fields, with targeted transport of drugs and medical imaging probably being the most important.
Currently we focus also on the study of mesoscale behavior of low molar mass (nonpolymeric) compounds, i.e. behavior on scales larger than molecular but smaller than macroscopic. It shows up that solubility in nature is achieved not only by well known “like likes like” or “like dissolves like” based on molecular solvation but also on mesoscale solubilization of dislike compounds characterized in that the solubility (homogeneous distribution over the whole volume of the system) is achieved on a mesoscale level, via nanoparticles or nanodroplets typically ranging in size from tens to hundreds of nanometers. An interesting group among low molar mass compounds represents in this respect gases. There is currently a vivid discussion in the scientific community whether stable bulk nanobubbles (nanobubbles in bulk liquids, not at interfaces) can exist and at which circumstances. Our department is also involved in this area of research. Nanobubbles are interesting because of both the basic research aspect and the applied research (numerous applications, especially in bio imaging).


The principal methods used in our department involve laser light scattering (static, dynamic and electrophoretic), making use of several properties of laser radiation such as small wavelengths of approximately hundreds of nanometers, monochromaticity and coherence. Static scattering provides structural information in the range of approximately 20 nm to microns, dynamic scattering carried out in the form of photon correlation spectroscopy gains information on dynamic processes with relaxation times in the range of many orders (from the submicrosecond range to seconds) and indirect structural information from 1 nm. The electrophoretic scattering method is used for measuring the dynamics of molecules and nanoparticles in electric fields, and provides information on charges which are considerably different from so-called chemical charges (expected on the basis of chemical structure) due to various physical effects. In colloid science, electrophoretic light scattering provides information on zeta potentials which are crucial for colloidal stability. These scattering techniques in our lab are complemented by Asymmetric-Flow Field-Flow Fractionation coupled with multiangle light scattering instrument. This method enables measurement of detailed and accurate size and mass distributions of complex samples comprising several species. Another approach is visualization of nanoobjects via ultramicroscopy and subsequent determination of size distributions via analyzing their diffusive motion via Nanoparticle Tracking Analysis. A unique method called Incremental Centrifugation Coupled with Light Scattering was developed in our laboratory and is capable of assessing densities of nanoparticles and nanodroplets of unknown origin and unknown concentration.
 Contact
Contact Intranet
Intranet SK
SK