International
| SUPRA-SIGHT – Štúdium štruktúry a dynamiky supramolekulárnych amyloidných štruktúr jednomolekulovou mikroskopiou a modelovaním pomocou strojového učenia | |
| Exploring Protein Amyloid Superstructures and Dynamics Using Single-Molecule Microscopy with the Deep-Learning Modeling | |
| Program: | JRP |
| Project leader: | RNDr., Ing. Šipošová Katarína, PhD. |
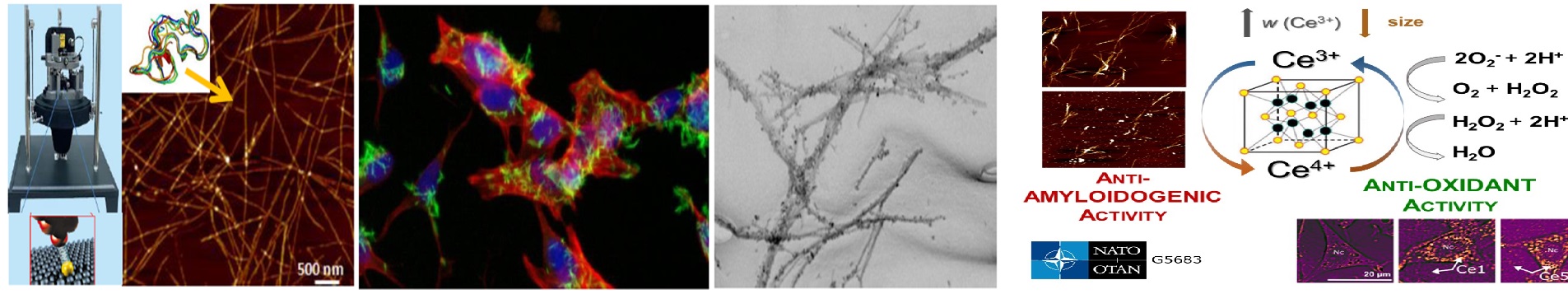
| Annotation: | Protein self-assembly processes independent of external energy sources and unlimited in-dimensional scaling have become a very promising approach for developing new materials with unprecedented structural and functional features. The best examples of self-assembled materials can be found in nature, including functional and pathological amyloid fibrils. Motivated by this, the proposal is aimed at basic as well as applied research and is represented by two major goals: a) anti-amyloid study – focusing on pathological amyloid aggregation connected with neurodegenerative diseases that will optimize the application of newly prepared compounds/particles in the treatment of amyloid-related diseases and enable imaging of amyloid formation/decomposition; b) fabrication of protein superstructures by chemical appending of functional ligands onto self-assembling peptides or proteins lays the foundation for developing new materials with unprecedented structural and functional features.Therefore, the development of the single-molecule orientation and localization microscopy (SMOLM), together with the deep-learning modeling for the localization and reconstruction of SMOLM images will help rapidly perfect the high-content analysis of the structural-morphological features not only the pathological amyloid aggregates but also superstructures consisting of amyloid scaffolds and connected bio-structures (DNA) and ligands. Moreover, the single-molecule orientation and localization microscopy could significantly contribute to understanding not only the process of protein self-assembly but also the mechanism of the binding and release of biologically active agents, formation and reorganization of multiple protein arrangements upon interaction with biologically relevant ligands/molecules. |
| Duration: | 1.1.2025 – 31.12.2027 |
| PHOTON – Príprava a charakterizácia proteínových hydrogélov s 3D zobrazovaním pomocou pokročilej nelineárnej optickej mikroskopie | |
| Fabrication and Characterization of Protein-based Hydrogels with Rapid 3D Imaging via Advanced Nonlinear Optical Microscopy | |
| Program: | Bilateral – other |
| Project leader: | RNDr., Ing. Šipošová Katarína, PhD. |
| Annotation: | Proteins displaying remarkable structural and functional characteristics such as biocompatibility, biodegradability, abundance, and reduced ability to induce immune and tissue inflammatory responses possess great potential for the manufacture of hydrogels. In addition, all proteins have the potential to be cross-linked, therefore by employing physical, chemical, and enzymatic treatments, proteins have innate benefits for hydrogel-forming. Amyloidal proteins are a category of programmable self-assembled macromolecules, and their assembly and owing to the programmability of the self-assembly of amyloidal structures, the consequent nanostructure can be manipulated rationally. Moreover, self-assembly allows the co-assembly of two or more types of building blocks, resulting in increasingly structurally complex nano-assemblies that may have physical and chemical properties distinct from those of the original mono-structures. Therefore, in the crosslinking-controlled strategy, by changing the nature and concentration of proteins, the resulting physical-chemical properties of amyloid-inspired hydrogel can be rationally tuned. Motivated by this, the proposal is aimed at basic as well as applied research and is represented by two major goals: a) preparation of single (one)-type amyloid-based hydrogels; b) fabrication of mixed protein-hydrogels by entrapping DNA-protein hybrids that could lead to the developing new materials with unprecedented structural and functional features; (c) the application of advanced nonlinear optical microscopy for rapid 3D multiphoton fluorescence imaging and second harmonic generation imaging to investigate hydrogel structures; and (d) the integration of deep learning-based image enhancement techniques to improve 3D image quality and capture detailed structural information. |
| Duration: | 1.7.2025 – 30.6.2027 |
| Hybrid DNA-functionalized fibrils as nanostructured material for bioanalytical applications | |
| Hybrid DNA-functionalized fibrils as nanostructured material for bioanalytical applications | |
| Program: | Mobility |
| Project leader: | RNDr., Ing. Šipošová Katarína, PhD. |
| Duration: | 1.1.2024 – 31.12.2025 |
| Hyp4Amy – Ultrazvukom a magneticky indukovaná hypertermia ako liečebná modalita pre amyloidné ochorenia | |
| Ultrasound- and Magnetic-induced Hyperthermia as a Treatment Modality for Amyloid-related Diseases | |
| Program: | Bilateral – other |
| Project leader: | RNDr., Ing. Šipošová Katarína, PhD. |
| Annotation: | Nanoparticles (NPs), including those with magnetic properties, have attracted significant scientific interest due totheir applications in various fields of science and medicine. As a consequence, several promising treatmentprocedures have been developed, such as controlled and local drug delivery and release, photo- and sonodynamictherapy, and thermal therapy. NPs can also be used as biosensors or imaging contrast agents. Additionally, theyare being studied for their potential role in amyloidogenic diseases. Although great efforts have been made tounderstand the pathogenesis of these diseases and design effective therapy, there is still no treatment for amyloid -related diseases. A possible alternative non-pharmacological option for targeting cross -using energy generated by magnetic NPs as a result of applied magnetic field and/or ultrasound to disrupt largeamyloid structures (plaques) into smaller fragments. This proposal is aimed at basic as well as applied researchthat will help perfect the application of magnetic NPs in the treatment of amyloid-related diseases via thermal andultrasound-induced processes and enable tomography imaging of amyloid formation/decomposition. Efficaciousdrug/NPs deposition in the target site and operated activation of therapeutic and imaging agents reduce treatmenttime, while avoiding adverse damage and side effects resulting from systemic administration. The collaboration willnot only promote the bilateral exchange of knowledge, technology and experience, but also may accelerate thetranslation between biophysical/biochemical science and clinical study. |
| Duration: | 1.1.2024 – 31.12.2025 |
| Innovative water-soluble phytomaterial inhibitors for Alzheimer’s and Parkinson’s disease prevention | |
| Innovative water-soluble phytomaterial inhibitors for Alzheimer’s and Parkinson’s disease prevention | |
| Program: | Horizon 2020 |
| Project leader: | MUDr. Musatov Andrey, DrSc. |
| Duration: | 1.10.2022 – 28.2.2025 |
National
| SelfNano – Programovateľné samo-usporiadanie hybridných DNA-proteín nanosystémov pre kontrolovateľné viazanie a uvoľnovanie biologicky aktívnych látok | |
| Programmable self-organization of hybrid DNA-protein nanosystems for controlled binding and release of biologicals | |
| Program: | SRDA |
| Project leader: | RNDr., Ing. Šipošová Katarína, PhD. |
| Annotation: | Protein self-assembly is a process based on autonomous, non-covalent interactions between distinct building blocks without requirement of external energy sources. The possibility of chemical appending of functional ligands onto self-assembling peptides or proteins lays the foundation for developing new materials with unprecedented structural and functional features. Especially using sequence addressable DNAs, the synergistic combination into DNA-protein self-assembling systems, may lead to unique and sophisticated functional hybrid nanostructures, which are highly programmable and display remarkable features that create new opportunities to build materials on the nanoscale. Inspired by the unique ability of proteins to self-assemble into amyloid fibrils, we plan to use recombinant spider silk eADF4(C16) protein, insulin, Aβ peptide and lysozyme in order to demonstrate the versatility of the concept of DNA-assisted self-organization of higher-order fibrillar structures. We will explore two dynamic association modes, the temperature-controlled hybridization event of short overlapping DNA sequences and the highly specific DNA-aptamer-to-ligand binding controlled by the ligand affinity. Generally, we foresee the feasibility of the proposed nanofibrillar systems mate of DNA-protein hybrids for the construction of nanostructured materials in biomedical research for binding and release of biologically active agents, formation of multiple protein arrangements for efficient enzymatic cascades or even dyes positioning for efficient light harvesting systems. |
| Duration: | 1.7.2024 – 30.6.2028 |
| APBC – Konzorcium pokročilých proteínových biotechnológií: Model pre podporu ekonomického rastu a zmiernenie úniku mozgov na východnom Slovensku | |
| The Advanced Protein Biotechnology Consortium: A Model for Fostering Economic Growth and Mitigating Brain Drain in Eastern Slovakia | |
| Program: | Plán obnovy EÚ |
| Project leader: | doc. RNDr. Gažová Zuzana, DrSc. |
| Duration: | 1.3.2025 – 31.8.2027 |
 Contact
Contact Intranet
Intranet SK
SK