International
| ML4NGP – Neglobulárne proteíny v ére strojového učenia | |
| Non-globular proteins in the era of Machine Learning | |
| Program: | COST |
| Project leader: | RNDr. Bednáriková Zuzana, PhD. |
| Annotation: | The ML4NGP Action aims to establish an interdisciplinary pan-European network to favour interplay between experiments and computation, fostering experimental frameworks designed to provide information to computational methods, and novel computational methods developed, trained and benchmarked with experimental data. ML4NGP will enhance the primary experimental data generation (WG1), promote integrative structural biology approaches (WG2), benchmark the state-of-the-art ML methods (WG3) and improve the functional characterization of NGPs (WG4). The Action will support its scientific objectives through policies that sustain free knowledge exchange, inclusiveness and training of young researchers who will lead future innovations in this field. |
| Project webpage: | https://www.cost.eu/actions/CA21160/ |
| Duration: | 25.10.2022 – 26.10.2026 |
| CHINMULTIHIT – Identifikácia malých molekúl z tradičných čínskych bylín na multicieľovú terapiu neurodegenerat ívnych ochorení a objasnenie mechanizmu účinku | |
| Discovery and Mechanism of Small Molecules from Traditional Chinese Herbs for Multitarget directed Therapy of Neurodegenerative Diseases | |
| Program: | Bilateral – other |
| Project leader: | doc. RNDr. Gažová Zuzana, DrSc. |
| Annotation: | Protein misfolding neurodegenerative diseases (NDDs), such as Alzheimer\’s and Parkinson\’s diseases, result inextensive cellular and neuronal loss within the central nervous system. Despite the distinct manifestations of thesedisorders, they share several common pathogenic mechanisms. Current pharmacological interventions areineffective. Moreover, the complexity of NDDs necessitates innovative therapeutic approaches. In this project, thenovel "multi-target-directed ligands" (MTDLs) strategy will be employed as it focuses on development of novelcompounds modulating multiple factors simultaneously. We will focus on Chinese herbs – Angelica sinensis,Ligusticum chuanxiong, and Cinnamomum cassia. The project aims to explore the anti-aggregation potential ofthese herbal extracts and their derivatives targeting both Aβ peptide and α -synuclein, showcasing neuroprotectiveand anti-inflammatory properties to pave the way for the development of novel t herapeutic strategies for NDDs.The project combines the expertise and experience of both research teams in the field of protein misfolding andNDDs pathology, allowing us to acquire complex data with the aid of complementary approaches, leading to thesuggestion of possible alternatives of therapy against these devastating diseases. Moreover, this collaborativeresearch partnership will present an excellent opportunity for both teams\’ young members to learn new techniquesin the well-equipped laboratories at East China University and IEP SAS and gain new experience by working in aninternational scientific environment. |
| Duration: | 1.7.2024 – 30.6.2026 |
| LEAPAB – Inhibícia agregácie A-beta peptidov proteínmi vyskytujúcimi sa počas neskorej embryogenézy: nový prístup liečby Alzheimerovej choroby | |
| Inhibition of A-beta Peptides Aggregation by Late Embryogenesis Abundant Proteins: A New Approach for Alzheimer’s Disease Treatment | |
| Program: | Bilateral – other |
| Project leader: | RNDr. Bednáriková Zuzana, PhD. |
| Annotation: | Alzheimer’s disease (AD) is the most common neurodegenerative disorder, sharing unclear pathophysiology and massive social costs. Today, more than 55 million people have been diagnosed with AD, which is forecast to increase more than twice by 2050. AD is tightly associated with the formation of deposits containing amyloid β (Aβ) peptide organized into insoluble amyloid fibrils. Despite numerous contemporary studies focused on reducing Aβ aggregation, a cure for AD has not yet been found. Our project intends to implement the elements of molecular mechanisms underlying the remarkable phenomenon of plant desiccation tolerance and develop a new Aβ anti-aggregation strategy. Late Embryogenesis Abundant proteins (LEAPs) are markedly induced upon desiccation and can stabilize the native structure of proteins and membranes by a mechanism that is not fully understood. The primary project aim is to investigate the structural properties of Ramonda serbica LEAPs and their interactions with Aβ peptides and aggregation. Firstly, we will recombinantly produce LEAPs with the highest potential to inhibit Aβ aggregation. Further, we will analyze LEAPs’ secondary structure under different conditions, focusing on their order-to-disorder transitions. The final aim is to identify Aβ/LEAPs interactions and assess the Aβ anti-aggregation potential of LEAPs in vitro, which will impact the development of new strategies for AD treatment. The collaborating teams involved in this project (Slovak – IEP SAS and Serbian – IMGGE) were selected based on their expertise in protein production, protein structure analysis and amyloid aggregation. The expertise transfer between partner laboratories will be ensured by mutual training of PhD and postdoc researchers involved in the project. |
| Duration: | 1.4.2024 – 31.12.2025 |
| Azobenzénové deriváty ako potenciálne terapeutiká pre Alzheimerovu chorobu | |
| Azobenzenes as potential Alzheimer\’s theranostic agents | |
| Program: | Mobility |
| Project leader: | RNDr. Bednáriková Zuzana, PhD. |
| Annotation: | Amyloid fibrils of amyloid β (Aβ) peptides are a neuropathological feature of Alzheimer\’s disease (AD). AD is one of the world\’s fastest-growing neurological diseases with substantial economic and societal impact, but no cure is currently available. Therefore, the exploration of novel treatment approaches is in high demand. The project\’s main objective is to study the ability of azobenzene molecules to affect targets associated with the amyloid cascade of AD pathogenesis. The project will employ the lever-like potential of azobenzene molecules to dissociate fibrillar aggregates of Aβ peptides and inhibit the proteolytic activity of β-secretase. We will integrate in vitro, in silico, and cells workflow to find a possible alternative therapy against this devastating disease. Moreover, this collaborative research partnership will present an excellent opportunity for both teams\’ young members to learn new techniques in the well-equipped laboratories at the Polish and Slovak Academies of Sciences and gain new experience by working in an international scientific environment. |
| Duration: | 1.1.2023 – 31.12.2024 |
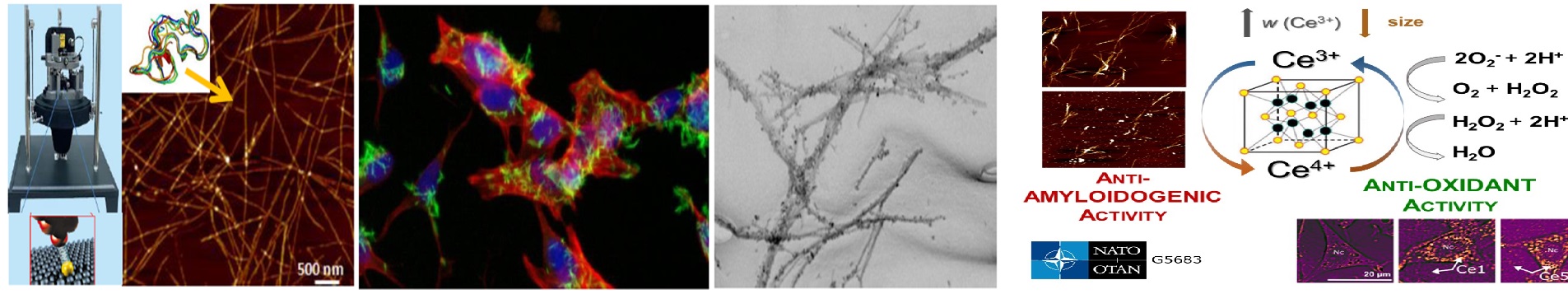
| ANOMATY – Interakcie amyloidných fibríl a nanočastíc pre biomedicínske, biochemické a inžinierske aplikácie | |
| Interactions of nanoparticles with amyloid fibrils: from therapy to nanomaterials | |
| Program: | Bilateral – other |
| Project leader: | doc. RNDr. Gažová Zuzana, DrSc. |
| Annotation: | Nanoparticles represent a powerful platform with a large potential for biomedicine and engineering applications.The formation of amyloid aggregates is unfavorable in vivo as they are associated with the pathogenesis of many human diseases, yet, amyloid fibrils have the potential to be engineered into novel materials. However, there is still little known about the interactions between amyloid fibrils and nanoparticles that can provide new enhanced NPs functions. The project aims to investigate the interaction of amyloid fibrils formed from native globular proteins and nanoparticles to utilize or enhance the NPs applications as catalysts in engineering applications or possible disaggregation agents to treat amyloid-related diseases (Alzheimer´s disease, diabetes mellitus). We will determine the relationship between amyloid fibrils (formed from lysozyme, insulin, and α -lactalbumin) and Au-, Ag- and Pd –nanoparticles with different surface chemistry (size, charge, functionalization). Moreover, we will perform a systematic study of globular proteins\’ propensity to form amyloid fibrils with controlled properties. The proposed objectives will be achieved by combining experimental techniques with computational methods routinely used in respective scientific teams. Moreover, the data about fibrils\’ structural and physico-chemical properties might fill the empty spaces in a big biology puzzle – pathophysiology of amyloid-related diseases. |
| Duration: | 1.1.2022 – 31.12.2023 |
| Stabilita a agregácia globulárnych proteínov v prítomnosti biokompatibilných iónových kvapalín | |
| Stability and aggregation of globular proteins in the presence of biocompatible ionic liquids | |
| Program: | Mobility |
| Project leader: | RNDr. Fedunová Diana, PhD. |
| Duration: | 1.1.2021 – 31.12.2022 |
| CHINMEDAMY – Identifikácia a mechanizmus účinku malých molekúl využívaných v tradičnej čínskej medicíne na liečbu Alzheimerovej choroby | |
| Discovery and Mechanism of Small Molecule Compounds from Traditional Chinese Medicine for treatment of Alzheimer \’s Disease | |
| Program: | Bilateral – other |
| Project leader: | doc. RNDr. Gažová Zuzana, DrSc. |
| Duration: | 1.1.2018 – 31.12.2019 |
| NGP-NET – Neglobulárne proteíny – od sekvencie ku štruktúre, funkcii a aplikácii v molekulárnej fyziopatológii | |
| Non-globular proteins – from sequence to structure, function and application in molecular physiopathology | |
| Program: | COST |
| Project leader: | doc. RNDr. Gažová Zuzana, DrSc. |
| Annotation: | Non-globular proteins (NGPs) encompass different molecular phenomena that defy the traditional sequence-structure-function paradigm. NGPs include intrinsically disordered regions, tandem repeats, aggregating domains, low-complexity sequences and transmembrane domains. Although growing evidence suggests that NGPs are central to many human diseases, functional annotation is very limited. It was recently estimated that close to 40 of all residues in the human proteome lack functional annotation and many of these are NGPs. While a better understanding of NGPs is crucial to fully comprehend human molecular physiopathology, progress has been hampered so far by the lack of a systematic approach to their study.This Action Proposal aims to create a pan-European scientific network of groups that work on NGPs to strengthen, focus and coordinate research in this field. It proposes to develop a novel classification of NGPs by consensus among interested experts that will be showcased on a newly developed web site, along with meetings, training schools and scientific missions on NGP-related topics. |
| Project webpage: | http://www.cost.eu/COST_Actions/bmbs/BM1405 |
| Duration: | 27.7.2015 – 25.3.2019 |
| Účinok malých molekúl a nanočastíc na amyloidnú agregáciu poly/peptidov | |
| Effect of small molecules and nanoparticles on amyloid aggregation of poly/peptides | |
| Program: | Bilateral – other |
| Project leader: | doc. RNDr. Gažová Zuzana, DrSc. |
| Annotation: | This project is aimed at examining the self-assembly of proteins into amyloid aggregates, one of the hallmarks of AD and other amyloidosis. Accordingly, there is a considerable world-wide interest to identify molecular entities that can influence the amyloid aggregation in order to facilitate the drug development for amyloid diseases. The main goals of the project are to estimate the conditions required for promoting protein misfolding, to determine the cytotoxicity of amyloid aggregates, and to identify the compounds (e.g. small molecules and nanoparticles) that are able to inhibit protein aggregation using in vitro and in silico methods. The bilateral collaboration will allow to combine expertise and experience of both partners in the field of protein aggregation and acquire complex data with aid of complementary approaches, leading to a better understanding of amyloid aggregation mechanisms. The use of equipment provided by both institutions will offer a solid background for team members in order to publish their results at conferences and in journals. Moreover, this collaborative research partnership will present an excellent opportunity for both teams’ young members to learn new techniques in the well-equipped laboratories at NTU and SAS and work as an international scientific research group. |
| Duration: | 11.1.2016 – 31.12.2018 |
| Amyloidná agregácia proteínov na hybridných povrchoch | |
| Amyloid aggregation of proteins in hybrid interfaces | |
| Program: | Inter-academic agreement |
| Project leader: | doc. RNDr. Gažová Zuzana, DrSc. |
| Annotation: | The aim of the project is a study of the mechanism of aggregation of the different amyloid proteins induced by interaction with various surfaces. The main focus will be concern on the characterization of protein aggregation induced by protein interaction with solid interfaces with various geometrical as well as the chemical properties of the surfaces. We will investigate the role of the surface forces and dimensionality of nanoparticles as agents that accelerate or prevent amyloid fibrillization of proteins. Moreover, effect of the interface polarity, substrate roughness or geometrical constraints will be examined. We will compare the morphological properties of amyloid aggregates formed in solution and at solid interface. |
| Duration: | 11.1.2016 – 31.12.2017 |
| ChinherbAD – Využitie multitargetových nízkomolekulových látok z tradičných čínskych bylín pri liečbe Alzheimerovej choroby | |
| The multitarget low molecular compounds from traditional Chinese herbs in treatment of Alzheimer´s disease | |
| Program: | Bilateral – other |
| Project leader: | doc. RNDr. Gažová Zuzana, DrSc. |
| Annotation: | Alzheimer\’s disease (AD) is a devastating neurodegenerative disorder of multifactorial nature characterized by neuroinflammation, decreasing the level of the neurotransmitter acetylcholine as well as formation of amyloid Abeta peptide plaques and neurofibrillary tangles containing abnormally posttranslationally modified tau protein. There is no effective treatment for AD so far and none of the clinically tested drugs have any feasibility to stop, substantially delay or reverse the progressive consequences of this disease. Accordingly, there is a considerable world-wide interest to facilitate the drug development for AD. Recently, the strategy of using the multi-targeted ligands seems to be the most attractive for developing effective therapy for Alzheimer´s disease due to ability of these compounds interacts with multiple targets responsible for the disease pathogenesis. The main goal of this project is to investigate multi-target responsibility of compounds including extracts from traditional Chinese herbs as potential therapeutic agents for AD. Using in vitro, in vivo and in silico methods we will study ability of these compounds to affect the neuroinflammation, to inhibit acetylcholinesterase activity, and amyloid aggregation of Abeta peptide. The bilateral collaboration will allow the both research groups to combine their expertise and experience in the field of pathology of Alzheimer´s disease. The complementary approach allows obtain more complex data leading to suggestion of possible alternatives of therapy against this devastating disease. Moreover, the project will also enable mutual utilization of equipment provided by both institutions. At the same time the young members of project\’s team will have the opportunity to learn new techniques in well-equipped laboratories at SAS and East China University of Science and Technology and also work in the international scientific team. |
| Duration: | 1.1.2016 – 31.12.2017 |
National
| Kapitálový booster pre schémy na podporu výskumu a vývoja (Viaczložkové ligandy ako modulátory cieľov spojených s patogenézou Alzheimerovej choroby) | |
| – | |
| Program: | Plán obnovy EÚ |
| Project leader: | doc. RNDr. Gažová Zuzana, DrSc. |
| Duration: | 1.7.2024 – 30.6.2025 |
| NANOVIR – Nanočastice pre riešenie diagnosticko-terapeutických problémov s COVID-19 (NANOVIR) | |
| – | |
| Program: | Štrukturálne fondy EÚ Výskum a inovácie |
| Project leader: | Ing. Závišová Vlasta, PhD. |
| Project webpage: | https://websrv.saske.sk/uef/veda-a-vyskum/projekty-v-ramci-opvai/nanovir/ |
| Duration: | 3.3.2021 – 30.6.2023 |
| BIOVID-19 – Vývoj biomodelov pre zlepšenie hodnotenia účinnosti liekov a látok, ktoré majú potenciál pri liečbe COVID-19 (BIOVID-19) | |
| – | |
| Program: | Štrukturálne fondy EÚ Výskum a inovácie |
| Project leader: | Ing. Koneracká Martina, CSc. |
| Project webpage: | https://websrv.saske.sk/uef/veda-a-vyskum/projekty-v-ramci-opvai/biovid-19/ |
| Duration: | 29.6.2021 – 30.6.2023 |
| MULTIHIT – Multifunkčné inhibítory poly/peptidov spojených s Alzheimerovou chorobou | |
| Multi-target inhibitors of poly/peptides associated with Alzheimer´s disease | |
| Program: | SRDA |
| Project leader: | doc. RNDr. Gažová Zuzana, DrSc. |
| Duration: | 1.7.2019 – 1.6.2023 |
| LEAPSynPD – Inhibícia agregácie α-synukleínu pomocou LEA proteínov: nový prístup pre liečbu Parkinsonovej choroby | |
| Inhibition of α-Synuclein Aggregation by LEA Proteins: A New Approach for Parkinson’s Disease Treatment | |
| Program: | SRDA |
| Project leader: | doc. RNDr. Gažová Zuzana, DrSc. |
| Annotation: | Intracellular protein aggregates mostly composed of α-Synuclein (α-Syn) are a pathological hallmark of Parkinson’s disease (PD), one of the world’s fastest growing neurological disorders. Recently, no efficient cure is available, therefore the exploration of novel approaches towards the development of a radically new therapy is urgently required. The potential of LEA proteins to inhibit α-Syn aggregation will be studied with aim to structurally characterized the interactions of LEA proteins with α-Syn and provide top LEAP candidates with significant anti-aggregation potential. |
| Duration: | 1.1.2022 – 31.1.2022 |
| Samousporiadanie poly/peptidov do amyloidných agregátov – mechanizmus, inhibícia a cytotoxicita | |
| Self-assembly of poly/peptides into amyloid aggregates – mechanism, inhibition and cytotoxicity | |
| Program: | VEGA |
| Project leader: | doc. RNDr. Gažová Zuzana, DrSc. |
| Annotation: | Amyloid supramolecular complexes formed by poly/peptides are the most prevalent naturally occurring self-assembling systems. Formation of such complexes affects function of poly/peptides and is associated with more than 30 serious amyloid-related diseases such as Alzhemer’s disease or diabetes mellitus. The exactmechanism of amyloid self-assembly of poly/peptides is not known yet and no effective treatment of amyloidosis is established. The goal of the project is to study the mechanism of the amyloid aggregation of poly/petides with different native structures and identification of inhibitors of poly/peptide self-assembly since the inhibition of amyloid formation is one of the possible therapeutic approaches against amyloid-related diseases. We will focus on the determination of the correlation between the morphology of amyloid aggregates and their cytotoxicity as well as on the relationship between structure of the effective inhibitors and their anti-amyloid activity. |
| Duration: | 1.1.2017 – 31.12.2020 |
 Contact
Contact Intranet
Intranet SK
SK