International
| Stabilita a agregácia globulárnych proteínov v prítomnosti biokompatibilných iónových kvapalín | |
| Stability and aggregation of globular proteins in the presence of biocompatible ionic liquids | |
| Program: | Mobility |
| Project leader: | RNDr. Fedunová Diana, PhD. |
| Duration: | 1.1.2021 – 31.12.2022 |
| NGP-NET – Neglobulárne proteíny – od sekvencie ku štruktúre, funkcii a aplikácii v molekulárnej fyziopatológii | |
| Non-globular proteins – from sequence to structure, function and application in molecular physiopathology | |
| Program: | COST |
| Project leader: | doc. RNDr. Gažová Zuzana, DrSc. |
| Annotation: | Non-globular proteins (NGPs) encompass different molecular phenomena that defy the traditional sequence-structure-function paradigm. NGPs include intrinsically disordered regions, tandem repeats, aggregating domains, low-complexity sequences and transmembrane domains. Although growing evidence suggests that NGPs are central to many human diseases, functional annotation is very limited. It was recently estimated that close to 40 of all residues in the human proteome lack functional annotation and many of these are NGPs. While a better understanding of NGPs is crucial to fully comprehend human molecular physiopathology, progress has been hampered so far by the lack of a systematic approach to their study.This Action Proposal aims to create a pan-European scientific network of groups that work on NGPs to strengthen, focus and coordinate research in this field. It proposes to develop a novel classification of NGPs by consensus among interested experts that will be showcased on a newly developed web site, along with meetings, training schools and scientific missions on NGP-related topics. |
| Project webpage: | http://www.cost.eu/COST_Actions/bmbs/BM1405 |
| Duration: | 27.7.2015 – 25.3.2019 |
National
| Objasnenie počiatočných štádií amyloidnej agregácie proteínov – od mechanizmu k terapii | |
| Unraveling the early events of protein amyloid aggregation – from mechanism to therapy | |
| Program: | VEGA |
| Project leader: | doc. RNDr. Gažová Zuzana, DrSc. |
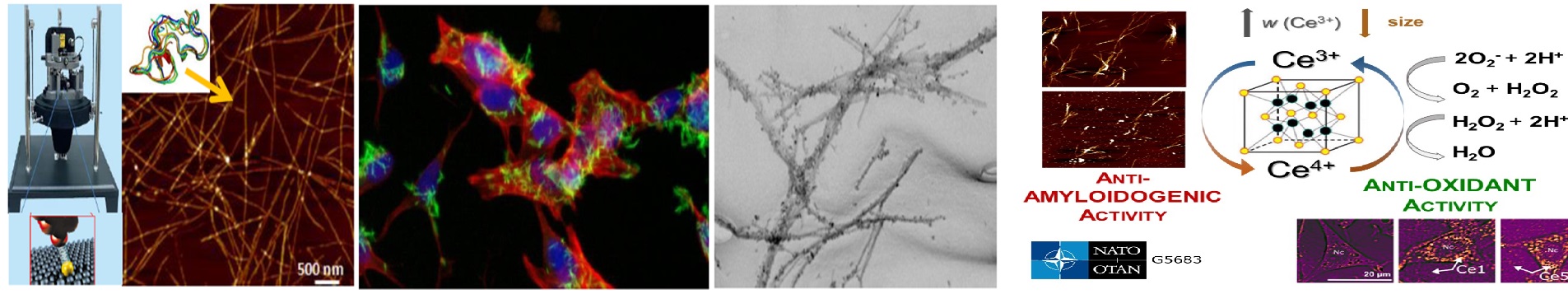
| Annotation: | Structural changes of poly/peptides leading to the formation of amyloid aggregates are associated with incurablediseases, like Alzheimer\’s disease or diabetes. While the general mechanisms of fibril formation and theircharacterization are well reported, the early events during poly/peptide fibrillation are still unclear. The project isfocused on understanding the early events mechanisms leading to the formation of pre-fibrillar (partially un/foldedintermediates, nuclei, oligomers) and fibrillar amyloid aggregates of selected globular and intrinsically disorderedproteins. Our focus will be the study of the kinetics of pre-fibrillar structures formation, their morphology, andcytotoxicity, under various experimental conditions, and in the presence of selected interacting partners (smallmolecules, nanoparticles). The obtained results will contribute to understanding the early events of amyloidaggregation and identifying the inhibitors with therapeutic potential for amyloid diseases. |
| Duration: | 1.1.2021 – 31.12.2024 |
| NANOVIR – Nanočastice pre riešenie diagnosticko-terapeutických problémov s COVID-19 (NANOVIR) | |
| – | |
| Program: | Štrukturálne fondy EÚ Výskum a inovácie |
| Project leader: | Ing. Závišová Vlasta, PhD. |
| Project webpage: | https://websrv.saske.sk/uef/veda-a-vyskum/projekty-v-ramci-opvai/nanovir/ |
| Duration: | 3.3.2021 – 30.6.2023 |
| MULTIHIT – Multifunkčné inhibítory poly/peptidov spojených s Alzheimerovou chorobou | |
| Multi-target inhibitors of poly/peptides associated with Alzheimer´s disease | |
| Program: | SRDA |
| Project leader: | doc. RNDr. Gažová Zuzana, DrSc. |
| Duration: | 1.7.2019 – 1.6.2023 |
| Nadmolekulárne komplexy proteínov – konformačné prechody, stabilita a agregácia | |
| Supramolecular complexes of proteins – conformational transitions, stability and aggregation | |
| Program: | VEGA |
| Project leader: | RNDr. Fedunová Diana, PhD. |
| Annotation: | Protein aggregation and self-assembly into supramolecular complexes occurs in various biological processes. Fibrillar aggregates – amyloids are hallmark of various diseases. Amyloid fibrils are part of physiological processes in cells and are also tested as novel biomaterials. The project is focused on study of the effect of two classes of cosolvents on amyloid aggregation of structurally different polypeptides – globular lysozyme and intrinsically disordered Aß peptide. The aim of the project is to find the relation between cosolvent properties and their effect on conformation, stability and kinetics of amyloid aggregation and morphology of obtained fibrils.Elucidation of these relations is important for the understanding of the mechanism of amyloid aggregation and can help to design new therapeutics against amyloid-related diseases, for identification of pathological structuralmotifs of fibrils as well as in biotechnological application of fibrils as novel materials. |
| Duration: | 1.1.2018 – 31.12.2021 |
| Samousporiadanie poly/peptidov do amyloidných agregátov – mechanizmus, inhibícia a cytotoxicita | |
| Self-assembly of poly/peptides into amyloid aggregates – mechanism, inhibition and cytotoxicity | |
| Program: | VEGA |
| Project leader: | doc. RNDr. Gažová Zuzana, DrSc. |
| Annotation: | Amyloid supramolecular complexes formed by poly/peptides are the most prevalent naturally occurring self-assembling systems. Formation of such complexes affects function of poly/peptides and is associated with more than 30 serious amyloid-related diseases such as Alzhemer’s disease or diabetes mellitus. The exactmechanism of amyloid self-assembly of poly/peptides is not known yet and no effective treatment of amyloidosis is established. The goal of the project is to study the mechanism of the amyloid aggregation of poly/petides with different native structures and identification of inhibitors of poly/peptide self-assembly since the inhibition of amyloid formation is one of the possible therapeutic approaches against amyloid-related diseases. We will focus on the determination of the correlation between the morphology of amyloid aggregates and their cytotoxicity as well as on the relationship between structure of the effective inhibitors and their anti-amyloid activity. |
| Duration: | 1.1.2017 – 31.12.2020 |
 Contact
Contact Intranet
Intranet SK
SK